Ati fundamental exam chapter 25 candile
Total Questions : 41
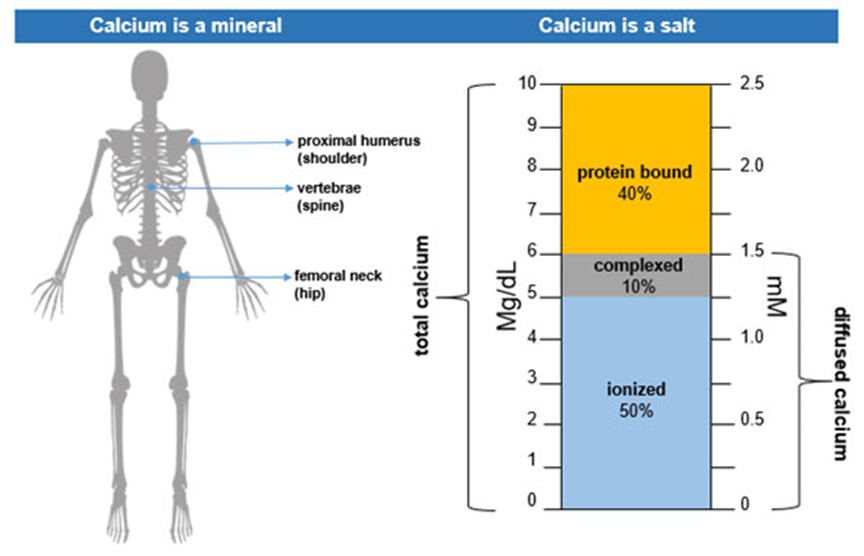
Showing 25 questions, Sign in for moreWhat is the normal range of serum calcium in adults?
Explanation
Choice A reason: This is a low value for serum calcium, which indicates hypocalcemia. Hypocalcemia can cause muscle spasms, tetany, seizures, and cardiac arrhythmias.
Choice B reason: This is also a low value for serum calcium, which indicates hypocalcemia. Hypocalcemia can cause muscle spasms, tetany, seizures, and cardiac arrhythmias.
Choice C reason: This is the normal range of serum calcium in adults. Calcium is essential for bone health, muscle contraction, nerve transmission, and blood clotting.
Choice D reason: This is a high value for serum calcium, which indicates hypercalcemia. Hypercalcemia can cause nausea, vomiting, constipation, confusion, lethargy, and kidney stones.
Lena Mason, who has diabetes, is admitted in a stuporous condition. Her blood gases show a pH of 7.33, PaCO2 of 40 mm Hg, and HCO3- of 20 mEq/L. What type of acid-base imbalance does this patient have?
Explanation
Choice A reason: This is incorrect because metabolic alkalosis is characterized by a high pH and a high HCO3-. The patient's pH and HCO3- are both low, indicating acidosis, not alkalosis.
Choice B reason: This is incorrect because respiratory alkalosis is characterized by a high pH and a low PaCO2. The patient's pH is low and PaCO2 is normal, indicating a metabolic problem, not a respiratory one.
Choice C reason: This is incorrect because respiratory acidosis is characterized by a low pH and a high PaCO2. The patient's pH is low, but PaCO2 is normal, indicating a metabolic problem, not a respiratory one.
Choice D reason: This is correct because metabolic acidosis is characterized by a low pH and a low HCO3-. The patient's pH and HCO3- are both low, indicating a metabolic disorder. The condition is uncompensated because the PaCO2 is normal, meaning the respiratory system is not compensating for the metabolic acidosis.
Which transport mechanism involves cellular energy?
Explanation
Choice A reason: This is incorrect because filtration is a passive transport mechanism that does not require cellular energy. Filtration is the movement of fluid and solutes across a membrane due to hydrostatic pressure.
Choice B reason: This is correct because active transport is a transport mechanism that requires cellular energy in the form of ATP. Active transport is the movement of molecules across a membrane against their concentration gradient, using carrier proteins.
Choice C reason: This is incorrect because diffusion is a passive transport mechanism that does not require cellular energy. Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration, until equilibrium is reached.
Choice D reason: This is incorrect because osmosis is a passive transport mechanism that does not require cellular energy. Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration.
The nurse points out that electrolytes are essential for health. Nonelectrolytes include:
Explanation
Choice A reason: This is incorrect because amino acids are electrolytes. Electrolytes are substances that dissociate into ions when dissolved in water and conduct electricity. Amino acids have both positive and negative charges and can form ions in solution.
Choice B reason: This is incorrect because magnesium is an electrolyte. Magnesium is a metal that forms positive ions (cations) when dissolved in water. Magnesium is important for muscle and nerve function, as well as bone health.
Choice C reason: This is incorrect because phosphates are electrolytes. Phosphates are compounds that contain the phosphate ion (PO4 3-), which is a negative ion (anion) in solution. Phosphates are involved in energy metabolism, acid-base balance, and bone formation.
Choice D reason: This is correct because glucose is a nonelectrolyte. Nonelectrolytes are substances that do not dissociate into ions when dissolved in water and do not conduct electricity. Glucose is a simple sugar that dissolves as a whole molecule in water. Glucose is the main source of energy for the body.
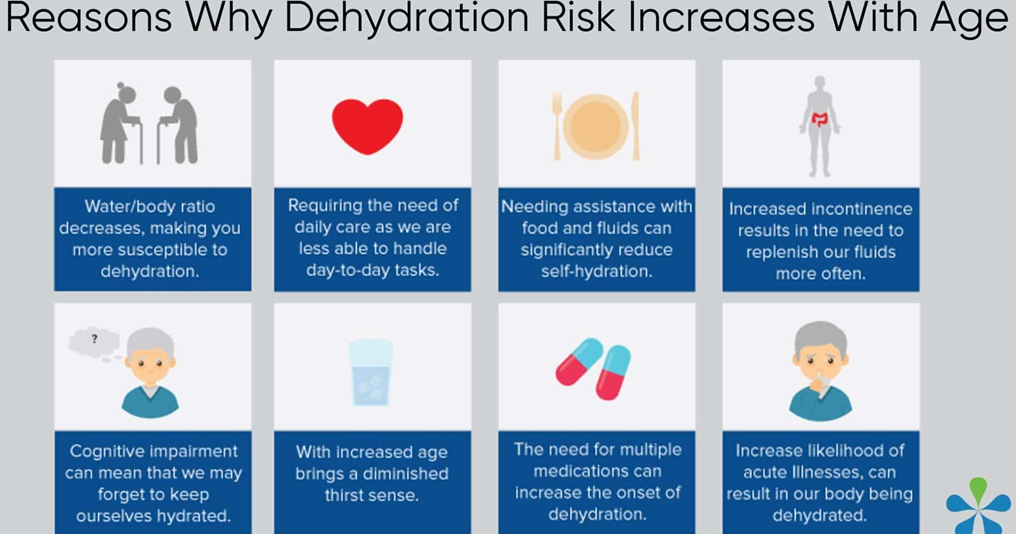
Which patient is at the highest risk for dehydration?
Explanation
Choice A: Elderly patients are at a higher risk for dehydration due to physiological changes that come with aging, such as decreased kidney function and physical changes to the body's water balance systems. Additionally, fever increases metabolic rate and fluid loss, and nausea and vomiting prevent adequate fluid intake, further increasing the risk of dehydration.
Choice B: While intentionally limiting fluid intake can lead to dehydration, the body's thirst mechanism in a healthy teenager is typically strong enough to prevent severe dehydration.
Choice C: Diarrhea can certainly lead to dehydration, but a young, otherwise healthy patient typically has a stronger ability to recover from fluid loss than an elderly patient.
Choice D: Infants are at a higher risk for dehydration than older children and adults due to their smaller body weight and higher turnover of water and electrolytes, but in this case, the elderly patient's multiple risk factors put them at a higher risk overall.
A nurse is reviewing the arterial blood gas (ABG) results of a client. The client's ABGs are:
pH 7.6
PaCO2 40 mm Hg
HCO3 32 mEq/L
Which of the following acid-base conditions should the nurse identify the client is experiencing?
Explanation
Choice A reason: This is correct because metabolic alkalosis is characterized by a high pH and a high HCO3-. The client's pH and HCO3- are both high, indicating a metabolic disorder. The condition is uncompensated because the PaCO2 is normal, meaning the respiratory system is not compensating for the metabolic alkalosis.
Choice B reason: This is incorrect because metabolic acidosis is characterized by a low pH and a low HCO3-. The client's pH and HCO3- are both high, indicating alkalosis, not acidosis.
Choice C reason: This is incorrect because respiratory alkalosis is characterized by a high pH and a low PaCO2. The client's pH is high, but PaCO2 is normal, indicating a metabolic problem, not a respiratory one.
Choice D reason: This is incorrect because respiratory acidosis is characterized by a low pH and a high PaCO2. The client's pH is high, and PaCO2 is normal, indicating a metabolic problem, not a respiratory one.
What is the normal range of serum sodium in adults?
Explanation
Choice A reason: This is incorrect because 120 to 140 mEq/L is a low range for serum sodium, which indicates hyponatremia. Hyponatremia can cause confusion, lethargy, seizures, and coma.
Choice B reason: This is correct because 135 to 145 mEq/L is the normal range of serum sodium in adults. Sodium is essential for fluid balance, nerve transmission, and muscle contraction.
Choice C reason: This is incorrect because 150 to 160 mEq/L is a high range for serum sodium, which indicates hypernatremia. Hypernatremia can cause thirst, dry mouth, agitation, and convulsions.
Choice D reason: This is incorrect because 165 to 175 mEq/L is a very high range for serum sodium, which indicates severe hypernatremia. Severe hypernatremia can cause irreversible brain damage and death.
The nurse is caring for a client with leukemia and notes that the client has poor skin turgor and flat neck and hand veins. The nurse suspects hypernatremia. Which sign/symptom would the nurse expect to note in this client if hypernatremia is present?
Explanation
Choice A reason: This is incorrect because polyuria is a sign of hyponatremia, not hypernatremia. Polyuria is the excessive production of urine, which can cause fluid loss and sodium dilution.
Choice B reason: This is correct because dry mucous membranes are a sign of hypernatremia. Dry mucous membranes are caused by dehydration, which can occur in hypernatremia due to fluid shifting from the intracellular to the extracellular space.
Choice C reason: This is incorrect because diarrhea is a sign of hyponatremia, not hypernatremia. Diarrhea is the frequent and watery passage of stool, which can cause fluid and electrolyte loss.
Choice D reason: This is incorrect because intense thirst is a sign of both hyponatremia and hypernatremia. Intense thirst is a result of the body's attempt to restore fluid balance and osmolarity.
Choice E reason: This is incorrect because vomiting is a sign of both hyponatremia and hypernatremia. Vomiting is a reflex action that expels the contents of the stomach, which can cause fluid and electrolyte loss or imbalance.
The nurse observed that a client with diabetic ketoacidosis is experiencing abnormally deep, regular, and rapid respirations. How would the nurse document this observation in the medical record?
Explanation
Choice A reason: This is incorrect because bradypnea is a term for slow breathing, usually less than 12 breaths per minute. The client is breathing rapidly, not slowly.
Choice B reason: This is correct because Kussmaul's respirations are a type of breathing pattern that is deep, regular, and rapid, usually more than 20 breaths per minute. Kussmaul's respirations are a sign of metabolic acidosis, which occurs in diabetic ketoacidosis due to the accumulation of ketones in the blood. The client is trying to exhale the excess carbon dioxide and lower the acidity of the blood.
Choice C reason: This is incorrect because Cheyne-Stokes respirations are a type of breathing pattern that is irregular, with periods of apnea (no breathing) alternating with periods of rapid breathing. Cheyne-Stokes respirations are a sign of cerebral dysfunction, such as stroke, brain injury, or coma.
Choice D reason: This is incorrect because Biot's respirations are a type of breathing pattern that is irregular, with periods of apnea (no breathing) interspersed with periods of normal breathing. Biot's respirations are a sign of brainstem damage, such as meningitis, encephalitis, or head trauma.
The nurse is caring for a client with respiratory insufficiency. The arterial blood gas (ABG) results indicate a pH of 7.50 and a PaCO2 of 30 mm Hg, and the nurse concludes that the client is experiencing respiratory alkalosis. Which additional laboratory value would the nurse expect to note in this client?
Explanation
Choice A reason: This is incorrect because sodium level of 145 mEq/L is within the normal range of 135 to 145 mEq/L. Sodium is not directly affected by respiratory alkalosis, but it may be altered by fluid balance or other conditions.
Choice B reason: This is incorrect because magnesium level of 1.3 mEq/L is within the normal range of 1.3 to 2.1 mEq/L. Magnesium is not directly affected by respiratory alkalosis, but it may be altered by renal function or other conditions.
Choice C reason: This is incorrect because phosphorus level of 3.0 mg/dL is within the normal range of 2.5 to 4.5 mg/dL. Phosphorus is not directly affected by respiratory alkalosis, but it may be altered by calcium balance or other conditions.
Choice D reason: This is correct because potassium level of 3.0 mEq/L is below the normal range of 3.5 to 5.0 mEq/L. Potassium is inversely related to hydrogen ions, which are decreased in respiratory alkalosis. As hydrogen ions move out of the cells to buffer the blood, potassium ions move into the cells to maintain electrical neutrality. This causes hypokalemia, or low potassium level.
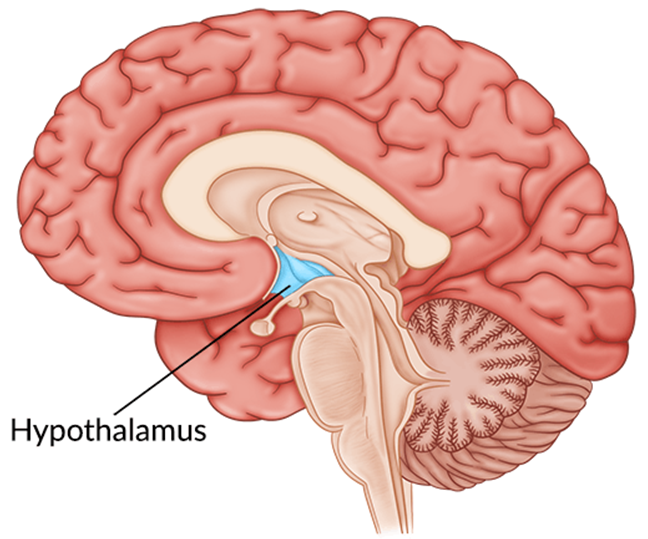
The thirst mechanism is located in the:
Explanation
Choice A reason: This is incorrect because the adrenal gland is not involved in the thirst mechanism. The adrenal gland is responsible for producing hormones such as cortisol, aldosterone, and adrenaline, which regulate stress response, blood pressure, and metabolism.
Choice B reason: This is incorrect because the cerebral cortex is not involved in the thirst mechanism. The cerebral cortex is the outer layer of the brain that is responsible for higher cognitive functions such as memory, language, and reasoning.
Choice C reason: This is incorrect because the pituitary gland is not directly involved in the thirst mechanism. The pituitary gland is a small gland at the base of the brain that produces hormones that control growth, reproduction, and metabolism. However, the pituitary gland does secrete antidiuretic hormone (ADH), which is regulated by the hypothalamus and affects water balance in the body.
Choice D reason: This is correct because the hypothalamus is the location of the thirst mechanism. The hypothalamus is a part of the brain that regulates many bodily functions such as temperature, appetite, sleep, and emotions. The hypothalamus also monitors the blood osmolarity and triggers the sensation of thirst when the blood is too concentrated.
Which of the following is the movement of a pure solvent (liquid) across a membrane?
Explanation
Choice A reason: This is incorrect because diffusion is the movement of solutes (dissolved substances) from an area of higher concentration to an area of lower concentration, until equilibrium is reached.
Choice B reason: This is incorrect because hydrostatic pressure is the force exerted by a fluid against a wall or a membrane. Hydrostatic pressure can drive the movement of fluid and solutes across a membrane, but it is not the movement itself.
Choice C reason: This is correct because osmosis is the movement of a pure solvent (liquid) across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration, until equilibrium is reached.
Choice D reason: This is incorrect because ATP is not a movement, but a molecule. ATP stands for adenosine triphosphate, which is the main source of energy for cellular processes. ATP can provide energy for some types of transport across membranes, such as active transport.
The nurse caring for a frail 92-year-old dehydrated patient should add to the plan of care:
Explanation
Choice A reason: This is incorrect because over-hydration is unlikely in a dehydrated patient. Over-hydration is a condition where the body has too much water, which can cause hyponatremia, edema, and cerebral swelling.
Choice B reason: This is incorrect because diarrhea is a cause, not a consequence, of dehydration. Diarrhea is the frequent and watery passage of stool, which can lead to fluid and electrolyte loss.
Choice C reason: This is incorrect because pulmonary congestion is also unlikely in a dehydrated patient. Pulmonary congestion is a condition where the lungs are filled with fluid, which can cause dyspnea, cough, and crackles.
Choice D reason: This is correct because fall is a potential complication of dehydration. Dehydration can cause confusion, dizziness, weakness, and hypotension, which can increase the risk of falling and injuring oneself.
A decreased serum pH causes a condition known as:
Explanation
Choice A reason: This is correct because acidosis is a condition where the serum pH is lower than the normal range of 7.35 to 7.45. Acidosis can be caused by an excess of acids or a loss of bases in the body, which can affect the function of various organs and systems.
Choice B reason: This is incorrect because equal bicarbonate is not a condition, but a term that describes the balance between bicarbonate (HCO3-) and carbonic acid (H2CO3) in the blood. Bicarbonate is a base that buffers the acids in the blood and maintains the pH. Equal bicarbonate means that the ratio of bicarbonate to carbonic acid is 20:1, which is the normal value.
Choice C reason: This is incorrect because neutral carbonic acid is not a condition, but a term that describes the pH of carbonic acid (H2CO3) in the blood. Carbonic acid is an acid that forms when carbon dioxide (CO2) dissolves in water. Neutral carbonic acid means that the pH of carbonic acid is 7.0, which is neither acidic nor basic.
Choice D reason: This is incorrect because alkalosis is a condition where the serum pH is higher than the normal range of 7.35 to 7.45. Alkalosis can be caused by a loss of acids or an excess of bases in the body, which can affect the function of various organs and systems.
If your patient has a higher than normal pH (alkalosis), you would expect to also see:
Explanation
Choice A reason: This is incorrect because low HCO3- and high PaCO2 are signs of metabolic acidosis, not alkalosis. Metabolic acidosis is a condition where the blood pH is lower than the normal range of 7.35 to 7.45, due to an excess of acids or a loss of bases in the body.
Choice B reason: This is incorrect because low PaCO2 and low HCO3- are signs of respiratory acidosis, not alkalosis. Respiratory acidosis is a condition where the blood pH is lower than the normal range of 7.35 to 7.45, due to impaired gas exchange or hypoventilation, which causes carbon dioxide to accumulate in the blood.
Choice C reason: This is correct because low PaCO2 and high HCO3- are signs of alkalosis. Alkalosis is a condition where the blood pH is higher than the normal range of 7.35 to 7.45, due to a loss of acids or an excess of bases in the body. There are two types of alkalosis: respiratory and metabolic. Respiratory alkalosis is caused by hyperventilation, which lowers the PaCO2 in the blood. Metabolic alkalosis is caused by vomiting, diuretics, or excessive intake of antacids, which raises the HCO3- in the blood.
Choice D reason: This is incorrect because high PaCO2 and high HCO3- are signs of compensation, not alkalosis. Compensation is a process where the body tries to restore the normal pH by adjusting the levels of PaCO2 and HCO3- in the opposite direction of the primary disorder. For example, if the patient has metabolic alkalosis, the respiratory system will try to compensate by retaining carbon dioxide and lowering the PaCO2. If the patient has respiratory alkalosis, the renal system will try to compensate by excreting bicarbonate and lowering the HCO3-.
An isotonic state exists within a patient's body fluids when the solute concentration of:
Explanation
Choice A reason: This is incorrect because intracellular fluid is greater than extracellular fluid in a hypertonic state, not an isotonic state. A hypertonic state is when the solute concentration of the extracellular fluid is higher than the intracellular fluid, which causes water to move out of the cells and shrink them.
Choice B reason: This is incorrect because extracellular fluid is less than intracellular fluid in a hypotonic state, not an isotonic state. A hypotonic state is when the solute concentration of the extracellular fluid is lower than the intracellular fluid, which causes water to move into the cells and swell them.
Choice C reason: This is correct because intracellular and extracellular fluid is equal in an isotonic state. An isotonic state is when the solute concentration of the extracellular fluid is the same as the intracellular fluid, which causes no net movement of water across the cell membrane.
Choice D reason: This is incorrect because interstitial fluid is less than the transcellular fluid in a situation of fluid imbalance, not an isotonic state. Interstitial fluid is the fluid that surrounds the cells, while transcellular fluid is the fluid that is contained in specialized cavities, such as cerebrospinal fluid, synovial fluid, or pleural fluid. The amount of transcellular fluid is normally very small compared to the interstitial fluid, but it can increase in certain conditions, such as edema, ascites, or hydrocephalus.
A patient has end-stage chronic obstructive pulmonary disease (COPD). Which acid-base imbalance would be predictable in a patient with COPD?
Explanation
Choice A reason: This is correct because respiratory acidosis is a condition where the blood pH is lower than the normal range of 7.35 to 7.45, due to impaired gas exchange or hypoventilation, which causes carbon dioxide to accumulate in the blood. COPD is a chronic lung disease that obstructs the airways and reduces the oxygen intake and carbon dioxide output. This leads to respiratory acidosis in the patient.
Choice B reason: This is incorrect because respiratory alkalosis is a condition where the blood pH is higher than the normal range of 7.35 to 7.45, due to hyperventilation, which lowers the carbon dioxide in the blood. COPD does not cause hyperventilation, but rather hypoventilation.
Choice C reason: This is incorrect because metabolic alkalosis is a condition where the blood pH is higher than the normal range of 7.35 to 7.45, due to a loss of acids or an excess of bases in the body. COPD does not affect the metabolic system directly, but rather the respiratory system.
Choice D reason: This is incorrect because metabolic acidosis is a condition where the blood pH is lower than the normal range of 7.35 to 7.45, due to an excess of acids or a loss of bases in the body. COPD does not affect the metabolic system directly, but rather the respiratory system.
The nurse is caring for a client with severe diarrhea. The nurse monitors the client closely, understanding that this client is at risk for developing which acid-base disorder?
Explanation
Choice A reason: This is incorrect because respiratory alkalosis is a condition where the blood pH is higher than the normal range of 7.35 to 7.45, due to hyperventilation, which lowers the carbon dioxide in the blood. Severe diarrhea does not cause hyperventilation, but rather dehydration and electrolyte imbalance.
Choice B reason: This is incorrect because metabolic alkalosis is a condition where the blood pH is higher than the normal range of 7.35 to 7.45, due to a loss of acids or an excess of bases in the body. Severe diarrhea does not cause a loss of acids or an excess of bases, but rather a loss of fluids and bicarbonate, which is a base that buffers the acids in the blood.
Choice C reason: This is correct because metabolic acidosis is a condition where the blood pH is lower than the normal range of 7.35 to 7.45, due to an excess of acids or a loss of bases in the body. Severe diarrhea causes a loss of fluids and bicarbonate, which is a base that buffers the acids in the blood. This leads to an accumulation of acids and a decrease in pH.
Choice D reason: This is incorrect because respiratory acidosis is a condition where the blood pH is lower than the normal range of 7.35 to 7.45, due to impaired gas exchange or hypoventilation, which causes carbon dioxide to accumulate in the blood. Severe diarrhea does not affect the respiratory system directly, but rather the metabolic system.
An isotonic solution contains equal solute concentration on both sides.
Explanation
An isotonic solution is a solution that has the same osmotic pressure as another solution, which means that the solute concentration on both sides of a semipermeable membrane is equal. This causes no net movement of water across the membrane, and the cells remain the same size and shape. An example of an isotonic solution is normal saline (0.9% sodium chloride), which is used to treat dehydration and fluid loss.
The nurse reviews the client's serum calcium level and notes that the level is 7.9 mg/dL. The nurse understands that which condition would cause this serum calcium level?
Explanation
Choice A reason: This is correct because prolonged bed rest can cause hypocalcemia, or low serum calcium level. Calcium is stored in the bones and is released into the blood when the bones are stressed by weight-bearing activities. When a person is on bed rest, the bones are not stimulated and the calcium remains in the bones, leading to a decrease in serum calcium level.
Choice B reason: This is incorrect because too much butter consumption does not affect the serum calcium level directly. Butter is a source of fat and calories, which can affect the cholesterol and triglyceride levels, but not the calcium level. However, too much butter consumption can cause obesity, which can increase the risk of osteoporosis and fractures.
Choice C reason: This is incorrect because hyperparathyroidism can cause hypercalcemia, or high serum calcium level. Hyperparathyroidism is a condition where the parathyroid glands produce too much parathyroid hormone (PTH), which regulates the calcium and phosphorus balance in the body. PTH stimulates the release of calcium from the bones into the blood, leading to an increase in serum calcium level.
Choice D reason: This is incorrect because excessive ingestion of vitamin D can also cause hypercalcemia, or high serum calcium level. Vitamin D is a fat-soluble vitamin that helps the body absorb calcium from the food and supplements. When a person takes too much vitamin D, the calcium absorption is increased and the serum calcium level rises.
What is the normal range of serum potassium level in adults?
Explanation
Choice A reason: This is correct because 3.5 to 5.0 mEq/L is the normal range of serum potassium level in adults. Potassium is an electrolyte that is important for nerve and muscle function, as well as acid-base balance.
Choice B reason: This is incorrect because 8.5 to 10.5 mg/dL is the normal range of serum calcium level in adults, not potassium. Calcium is an electrolyte that is involved in bone health, muscle contraction, and blood clotting.
Choice C reason: This is incorrect because 135 to 145 mEq/L is the normal range of serum sodium level in adults, not potassium. Sodium is an electrolyte that is essential for fluid balance, nerve transmission, and muscle contraction.
Choice D reason: This is incorrect because 1.8 to 2.6 mEq/L is the normal range of serum magnesium level in adults, not potassium. Magnesium is an electrolyte that is important for muscle and nerve function, as well as enzyme activity.
What is the normal range of serum chloride level in adults?
Explanation
Choice A reason: This is incorrect because 95-110 mg/dL is the normal range of serum phosphorus level in adults, not chloride. Phosphorus is an electrolyte that is involved in energy metabolism, acid-base balance, and bone formation.
Choice B reason: This is incorrect because 10-120 mEq/L is not a realistic range for any electrolyte level in the blood. The units of mEq/L indicate the concentration of ions, not the mass of the substance. The normal range of serum chloride level in adults is expressed in mEq/L, not mg/dL.
Choice C reason: This is correct because 96-106 mEq/L is the normal range of serum chloride level in adults. Chloride is an electrolyte that is important for fluid balance, acid-base balance, and nerve transmission.
Choice D reason: This is incorrect because 1.8-2.6 mEq/L is the normal range of serum magnesium level in adults, not chloride. Magnesium is an electrolyte that is important for muscle and nerve function, as well as enzyme activity.
Which may cause hyperkalemia?
Explanation
Choice A reason: Renal failure can cause hyperkalemia because the kidneys are unable to excrete excess potassium from the body. This can lead to high levels of potassium in the blood, which can affect the heart and muscles.
Choice B reason: Diarrhea can cause hypokalemia, not hyperkalemia, because it can lead to loss of potassium from the gastrointestinal tract. This can result in low levels of potassium in the blood, which can also affect the heart and muscles.
Choice C reason: Blood transfusion can cause hyperkalemia if the blood is old or hemolyzed, meaning that the red blood cells have broken down and released potassium into the plasma. This can increase the potassium levels in the recipient's blood.
Choice D reason: Diaphoresis, or sweating, can cause hypokalemia, not hyperkalemia, because it can lead to loss of potassium from the skin. This can also lower the potassium levels in the blood.
Which electrolyte imbalance is most likely to cause abdominal pain, urinary retention, and confusion?
Explanation
Choice A reason: Sodium (Na+) imbalance can cause neurological symptoms such as confusion, seizures, or coma, but not abdominal pain or urinary retention.
Choice B reason: Calcium (Ca2+) imbalance can cause abdominal pain, urinary retention, and confusion, as well as muscle weakness, bone pain, and cardiac arrhythmias. These signs are the result of an inadequate supply of calcium, which is essential for nerve and muscle function, as well as bone health.
Choice C reason: Chloride (Cl-) imbalance can cause acid-base disorders such as metabolic acidosis or alkalosis, but not abdominal pain, urinary retention, or confusion.
Choice D reason: Phosphates (PO4^3^-) imbalance can cause bone and muscle problems, such as rickets, osteomalacia, or tetany, but not abdominal pain, urinary retention, or confusion.
Choice E reason: Potassium (K+) imbalance can cause cardiac and neuromuscular symptoms, such as arrhythmias, palpitations, muscle weakness, or paralysis, but not abdominal pain, urinary retention, or confusion.
The nurse is caring for a client with a nasogastric tube that is attached to low suction. The nurse monitors the client closely for which acid-base disorder that is most likely to occur in this situation?
Explanation
Choice A reason: Respiratory alkalosis is caused by hyperventilation, which lowers the carbon dioxide levels in the blood and raises the pH. This is not likely to occur in a client with a nasogastric tube on low suction.
Choice B reason: Metabolic acidosis is caused by an excess of acids or a loss of bases in the body, which lowers the pH. This can occur in conditions such as diabetic ketoacidosis, renal failure, or diarrhea. This is not likely to occur in a client with a nasogastric tube on low suction.
Choice C reason: Respiratory acidosis is caused by hypoventilation, which raises the carbon dioxide levels in the blood and lowers the pH. This can occur in conditions such as chronic obstructive pulmonary disease, asthma, or sedative overdose. This is not likely to occur in a client with a nasogastric tube on low suction.
Choice D reason: Metabolic alkalosis is caused by a loss of acids or an excess of bases in the body, which raises the pH. This can occur in conditions such as vomiting, gastric suction, or diuretic use. This is the most likely acid-base disorder to occur in a client with a nasogastric tube on low suction, as the tube removes gastric acid from the stomach.
Sign Up or Login to view all the 41 Questions on this Exam
Join over 100,000+ nursing students using Nursingprepexams’s science-backend flashcards, practice tests and expert solutions to improve their grades and reach their goals.
Sign Up Now

