Life Science Fall 2023 Lab Exam 1
Total Questions : 46
Showing 25 questions, Sign in for moreWhich of the following is NOT a product of the light reaction?
Explanation
Choice A rationale: Carbon dioxide is not a product of the light reaction, but a reactant of the dark reaction. The light reaction does not use carbon dioxide, but water and light energy to produce ATP, NADPH, and oxygen¹.
Choice B rationale: NADPH is a product of the light reaction. It is an electron carrier that is reduced by the electrons from water in photosystem I. NADPH provides electrons and hydrogen for the dark reaction².
Choice C rationale: ATP is a product of the light reaction. It is an energy molecule that is synthesized by the enzyme ATP synthase using the proton gradient created by the electron transport chain. ATP provides energy for the dark reaction³.
Choice D rationale: Oxygen is a product of the light reaction. It is released as a by-product of the splitting of water in photosystem II. Oxygen is either used for cellular respiration or released into the atmosphere⁴.
Choice E rationale: Energy intermediates are not a specific product of the light reaction, but a general term for molecules that store energy or electrons, such as ATP and NADPH. Therefore, this choice is not incorrect, but less specific than choice A⁵.
Explanation
Choice A rationale: CO2 is not the source of oxygen produced by a plant, but a reactant of the dark reaction. The dark reaction uses CO2 and energy intermediates from the light reaction to produce glucose, a type of sugar. The dark reaction does not release any oxygen¹.
Choice B rationale: C6H12O6 is the chemical formula for glucose, which is the product of the dark reaction. Glucose is synthesized from CO2 and energy intermediates from the light reaction. Glucose does not produce any oxygen, but can be used by the plant for respiration or storage².
Choice C rationale: Glyceraldehyde-3-phosphate is an intermediate molecule in the dark reaction. It is formed from CO2 and energy intermediates from the light reaction, and then converted into glucose. Glyceraldehyde-3-phosphate does not produce any oxygen³.
Choice D rationale: H2O is the source of oxygen produced by a plant. In the light reaction, water is split by the energy from sunlight in photosystem II, releasing electrons, protons, and oxygen. The oxygen is either used for respiration or released into the air⁴.
Choice E rationale: O2 is the product of oxygen produced by a plant, not the source. O2 is released as a by-product of the splitting of water in photosystem II. O2 is either used for respiration or released into the air⁴.
Multiple Choice
Explanation
Choice A rationale: Microwaves are a type of electromagnetic radiation with low energy and long wavelengths. They are not absorbed by plants for photosynthesis, but rather pass through them or are reflected by them¹.
Choice B rationale: Infrared is a type of electromagnetic radiation with low energy and long wavelengths. It is not absorbed by plants for photosynthesis, but rather heats up the plant tissues or is reflected by them².
Choice C rationale: Gamma rays are a type of electromagnetic radiation with high energy and short wavelengths. They are not absorbed by plants for photosynthesis, but rather damage the plant cells or are blocked by the atmosphere³.
Choice D rationale: Ultraviolet is a type of electromagnetic radiation with high energy and short wavelengths. It is not absorbed by plants for photosynthesis, but rather harms the plant pigments or is filtered by the ozone layer⁴.
Choice E rationale: Visible light is a type of electromagnetic radiation with moderate energy and wavelengths. It is the only form of electromagnetic radiation that is absorbed by plants for photosynthesis, specifically by the pigments such as chlorophyll and carotenoids in the photosystems⁵. Visible light consists of a spectrum of colors, ranging from violet to red, and plants use different colors for different aspects of photosynthesis⁶.
Explanation
Choice A rationale: H2O is a reactant or input for the light reaction of photosynthesis. In the light reaction, water is split by the energy from sunlight in photosystem II, releasing electrons, protons, and oxygen. The electrons and protons are used to reduce NADP+ to NADPH and to synthesize ATP from ADP and Pi. The oxygen is either used for respiration or released into the air¹².
Choice B rationale: Oxygen is not a reactant or input for the light reaction of photosynthesis, but a product or output. Oxygen is released as a by-product of the splitting of water in photosystem II. Oxygen is either used for respiration or released into the air¹².
Choice C rationale: NADPH is not a reactant or input for the light reaction of photosynthesis, but a product or output. NADPH is an electron carrier that is reduced by the electrons from water in photosystem I. NADPH provides electrons and hydrogen for the dark reaction, which uses CO2 to produce glucose¹².
Choice D rationale: ATP is not a reactant or input for the light reaction of photosynthesis, but a product or output. ATP is an energy molecule that is synthesized by the enzyme ATP synthase using the proton gradient created by the electron transport chain. ATP provides energy for the dark reaction, which uses CO2 to produce glucose¹².
Choice E rationale: Carbon dioxide is not a reactant or input for the light reaction of photosynthesis, but a reactant or input for the dark reaction. The dark reaction uses CO2 and energy intermediates from the light reaction to produce glucose, a type of sugar. The dark reaction does not require light and can occur in the day or night¹².
Which best explains the role of plant pigments in photosynthesis?
Explanation
Choice A rationale: Plant pigments do not produce photon energy, but rather capture it from the sun. Photon energy is the energy carried by particles of light, called photons. Different types of electromagnetic radiation, such as visible light, have different amounts of photon energy depending on their wavelength¹.
Choice B rationale: Plant pigments absorb light energy and use it to initiate photosynthesis. Photosynthesis is the process by which plants convert light energy into chemical energy, stored in the bonds of sugar molecules. Plant pigments are specialized organic molecules, such as chlorophyll and carotenoids, that are found in the chloroplasts of plant cells. They absorb specific wavelengths of light and reflect others, giving plants their characteristic colors²³.
Choice C rationale: Plant pigments do not provide electrons, but rather transfer them to other molecules. Electrons are negatively charged subatomic particles that are involved in chemical reactions. In photosynthesis, plant pigments absorb light energy and use it to split water molecules, releasing electrons, protons, and oxygen. The electrons are then passed along an electron transport chain, generating a proton gradient that drives the synthesis of ATP, an energy molecule. The electrons are also used to reduce NADP+ to NADPH, an electron carrier⁴.
Choice D rationale: Plant pigments do not convert heat to electricity, but rather convert light to chemical energy. Heat and electricity are both forms of energy, but they are not directly involved in photosynthesis. Heat is the kinetic energy of molecules, while electricity is the flow of electrons or electric charge. Plant pigments absorb light energy and use it to drive the chemical reactions of photosynthesis, which produce sugar and oxygen as products⁵.
Choice E rationale: Plant pigments do not reduce NADP, but rather donate electrons to it. Reduction is a chemical reaction in which a molecule gains electrons, while oxidation is a chemical reaction in which a molecule loses electrons. NADP+ is an oxidized form of NADP, which stands for nicotinamide adenine dinucleotide phosphate. It is an electron carrier that accepts electrons from plant pigments in photosystem I, a complex of proteins and pigments in the thylakoid membrane of the chloroplast. The reduced form of NADP is NADPH, which carries electrons and hydrogen for the dark reaction of photosynthesis, which uses CO2 to produce glucose⁶.
Explanation
Choice A rationale: Chlorophyll is the molecule that traps the sun's energy and initiates the process of photosynthesis. Photosynthesis is the process by which plants convert light energy into chemical energy, stored in the bonds of sugar molecules. Chlorophyll is a green pigment that is found in the thylakoid membranes of the chloroplasts, the organelles where photosynthesis occurs. Chlorophyll absorbs red and blue light from the sun and reflects green light, giving plants their characteristic color. Chlorophyll also transfers the light energy to electrons, which are then used to split water molecules and generate ATP and NADPH, the energy intermediates for the dark reaction of photosynthesis¹².
Choice B rationale: ATP is not the molecule that traps the sun's energy, but an energy molecule that is synthesized by the light reaction of photosynthesis. ATP stands for adenosine triphosphate, and it consists of a nitrogenous base, a sugar, and three phosphate groups. ATP stores energy in the bonds between the phosphate groups, and releases energy when one of the bonds is broken, forming ADP (adenosine diphosphate) and Pi (inorganic phosphate). ATP provides energy for the dark reaction of photosynthesis, which uses CO2 to produce glucose, a type of sugar³⁴.
Choice C rationale: Chloroplast is not the molecule that traps the sun's energy, but the organelle where photosynthesis occurs. Chloroplast is a membrane-bound structure that is found in the cytoplasm of plant cells. Chloroplast contains its own DNA and ribosomes, and can divide independently of the cell. Chloroplast has two main parts: the stroma, which is the fluid-filled space inside the chloroplast, and the thylakoid, which is a system of flattened sacs that contain chlorophyll and other pigments. The light reaction of photosynthesis takes place in the thylakoid, while the dark reaction takes place in the stroma⁵⁶.
Choice D rationale: Glyceraldehyde-3-phosphate is not the molecule that traps the sun's energy, but an intermediate molecule in the dark reaction of photosynthesis. Glyceraldehyde-3-phosphate, also known as G3P, is a three-carbon sugar that is formed from CO2 and energy intermediates from the light reaction. G3P can be converted to glucose, which is the main product of photosynthesis, or to other organic molecules, such as amino acids, lipids, and nucleotides. G3P can also be recycled to regenerate the five-carbon starter molecule called ribulose, which is needed for the dark reaction to continue⁷⁸.
Choice E rationale: Rubisco is not the molecule that traps the sun's energy, but an enzyme that catalyzes the first step of the dark reaction of photosynthesis. Rubisco stands for ribulose-1,5-bisphosphate carboxylase/oxygenase, and it is the most abundant protein on Earth. Rubisco combines CO2 with ribulose, a five-carbon sugar, to form a six-carbon intermediate that splits into two molecules of G3P. Rubisco is also responsible for a wasteful process called photorespiration, in which it binds O2 instead of CO2, resulting in the loss of carbon and energy⁹ .
Explanation
Choice A rationale: Starch solution is not a protein solution, but a carbohydrate solution. Starch is a polysaccharide, which is a polymer of glucose molecules. Starch does not contain peptide bonds, which are the bonds that link amino acids in proteins. Therefore, starch solution would not react with the biuret reagent and would not produce a violet color.
Choice B rationale: Olive oil is not a protein solution, but a lipid solution. Olive oil is mainly composed of triglycerides, which are esters of glycerol and fatty acids. Olive oil does not contain peptide bonds, which are the bonds that link amino acids in proteins. Therefore, olive oil would not react with the biuret reagent and would not produce a violet color.
Choice C rationale: Albumin solution is a protein solution. Albumin is a globular protein that is found in blood plasma and egg white. Albumin contains many peptide bonds, which are the bonds that link amino acids in proteins. Therefore, albumin solution would react with the biuret reagent and would produce a violet color.
Choice D rationale: Distilled water is not a protein solution, but a pure solvent. Distilled water is water that has been purified by boiling and condensing. Distilled water does not contain any solutes, such as proteins, carbohydrates, or lipids. Therefore, distilled water would not react with the biuret reagent and would not produce a violet color.
Choice E rationale: Glucose solution is not a protein solution, but a carbohydrate solution. Glucose is a monosaccharide, which is a simple sugar. Glucose does not contain peptide bonds, which are the bonds that link amino acids in proteins. Therefore, glucose solution would not react with the biuret reagent and would not produce a violet color.
Explanation
Choice A rationale: Albumin solution is not a negative control, but a positive control for the test for protein. Albumin is a type of protein that reacts with the biuret reagent and produces a violet color. A positive control is used to confirm that the test works and gives a positive result when the substance is present³.
Choice B rationale: Starch solution is not a negative control, but a positive control for the test for starch. Starch is a type of carbohydrate that reacts with the iodine solution and produces a blue-black color. A positive control is used to confirm that the test works and gives a positive result when the substance is present³.
Choice C rationale: Glucose solution is not a negative control, but a positive control for the test for sugar. Glucose is a type of sugar that reacts with the Benedict's solution and produces a red-orange color. A positive control is used to confirm that the test works and gives a positive result when the substance is present³.
Choice D rationale: Olive oil is not a negative control, but a positive control for the test for lipids. Olive oil is a type of lipid that reacts with the Sudan III solution and produces a red color. A positive control is used to confirm that the test works and gives a positive result when the substance is present³.
Choice E rationale: Distilled water is a negative control for the tests for protein, lipids, sugars, and starch. Distilled water is a pure solvent that does not contain any of these substances. It does not react with any of the reagents and does not produce any color change. A negative control is used to confirm that there is no response to the reagent or the microorganism used in the test. It is used to set the baseline and verify that the detecting reagent is working properly³.
Explanation
Choice A rationale: Sudan IV is not a reagent for protein detection, but a reagent for lipid detection. Sudan IV is a red dye that binds to non-polar molecules, such as fats and oils. Sudan IV stains lipids red, while leaving water-soluble molecules, such as proteins, unstained¹.
Choice B rationale: Benedict's is not a reagent for protein detection, but a reagent for sugar detection. Benedict's is a blue solution that contains copper sulfate, sodium carbonate, and sodium citrate. Benedict's reacts with reducing sugars, such as glucose and fructose, and reduces the copper ions from blue to orange-red².
Choice C rationale: Biuret is a reagent for protein detection. Biuret is a blue solution that contains copper sulfate and sodium hydroxide. Biuret reacts with peptide bonds, which are the bonds that link amino acids in proteins. Biuret changes color from blue to violet when it binds to protein molecules³.
Choice D rationale: Iodine is not a reagent for protein detection, but a reagent for starch detection. Iodine is a brown solution that forms a complex with starch, a polysaccharide composed of glucose units. Iodine changes color from brown to blue-black when it interacts with starch molecules⁴.
The positive control for the iodine test was the
Explanation
Choice A rationale: Distilled water is not a positive control, but a negative control for the iodine test. Distilled water is a pure solvent that does not contain any starch or other carbohydrates. It does not react with the iodine solution and does not produce any color change. A negative control is used to confirm that there is no response to the reagent or the microorganism used in the test. It is used to set the baseline and verify that the detecting reagent is working properly³.
Choice B rationale: Olive oil is not a positive control, but a negative control for the iodine test. Olive oil is a lipid that does not contain any starch or other carbohydrates. It does not react with the iodine solution and does not produce any color change. A negative control is used to confirm that there is no response to the reagent or the microorganism used in the test. It is used to set the baseline and verify that the detecting reagent is working properly³.
Choice C rationale: Albumin solution is not a positive control, but a negative control for the iodine test. Albumin is a protein that does not contain any starch or other carbohydrates. It does not react with the iodine solution and does not produce any color change. A negative control is used to confirm that there is no response to the reagent or the microorganism used in the test. It is used to set the baseline and verify that the detecting reagent is working properly³.
Choice D rationale: Starch solution is a positive control for the iodine test. Starch is a polysaccharide that contains many glucose units linked by glycosidic bonds. Starch reacts with the iodine solution and produces a blue-black color. A positive control is used to confirm that the test works and gives a positive result when the substance is present³.
Choice E rationale: Glucose solution is not a positive control, but a negative control for the iodine test. Glucose is a monosaccharide that does not contain any glycosidic bonds. Glucose does not react with the iodine solution and does not produce any color change. A negative control is used to confirm that there is no response to the reagent or the microorganism used in the test. It is used to set the baseline and verify that the detecting reagent is working properly³.
Explanation
Choice A reason: Benedict's test is a test for the presence of reducing sugars, such as glucose or maltose, in a solution. The test involves adding Benedict's reagent, which is a blue solution of copper (II) sulfate, sodium carbonate, and sodium citrate, to the solution and heating it in a water bath. If reducing sugars are present, they reduce the copper (II) ions to copper (I) ions, which form a red, orange, or green precipitate of copper (I) oxide. The color and amount of the precipitate indicate the concentration of reducing sugars in the solution. ¹
Choice B reason: Brown paper test is a test for the presence of lipids, such as fats or oils, in a solution. The test involves placing a drop of the solution on a piece of brown paper and letting it dry. If lipids are present, they leave a translucent spot on the paper, which can be seen by holding the paper against a light source. The test is based on the fact that lipids are nonpolar and do not dissolve in water, but can dissolve in organic solvents and stain the paper. ²
Choice C reason: Biuret test is a test for the presence of proteins or peptides in a solution. The test involves adding Biuret reagent, which is a blue solution of copper (II) sulfate and sodium hydroxide, to the solution. If proteins or peptides are present, they form a complex with the copper (II) ions, which changes the color of the solution to violet or pink. The test is based on the fact that proteins and peptides have peptide bonds, which have nitrogen atoms that can coordinate with the copper (II) ions. ³
Choice D reason: Iodine test is a test for the presence of starch in a solution. The test involves adding iodine solution, which is a brown solution of iodine and potassium iodide, to the solution. If starch is present, it forms a complex with the iodine molecules, which changes the color of the solution to blue-black. The test is based on the fact that starch is a polysaccharide that has a helical structure, which can trap the iodine molecules inside. ⁴
Choice E reason: Wendelspecht test is a fictional test that does not exist in reality. It is a made-up name that has no meaning or relevance to the topic of this question. Therefore, it cannot be a valid answer.
Explanation
Choice A reason: Biuret test is a test for the presence of proteins or peptides in a solution. It involves adding Biuret reagent, which is a blue solution of copper (II) sulfate and sodium hydroxide, to the solution. If proteins or peptides are present, they form a complex with the copper (II) ions, which changes the color of the solution to violet or pink. ³
Choice B reason: Gram's iodine test is a test for the presence of starch in a solution. It involves adding iodine solution, which is a brown solution of iodine and potassium iodide, to the solution. If starch is present, it forms a complex with the iodine molecules, which changes the color of the solution to blue-black. ⁴
Choice C reason: Ninhydrin test is a test for the presence of amines or amino acids in a solution. It involves adding ninhydrin reagent, which is a purple solution of ninhydrin, to the solution. If amines or amino acids are present, they react with ninhydrin to produce a purple color, often called Ruhemann's purple. ¹
Choice D reason: Brown paper test is a test for the presence of lipids, such as fats or oils, in a solution. The test involves placing a drop of the solution on a piece of brown paper and letting it dry. If lipids are present, they leave a translucent spot on the paper, which can be seen by holding the paper against a light source. The test is based on the fact that lipids are nonpolar and do not dissolve in water, but can dissolve in organic solvents and stain the paper. ²
Choice E reason: Benedict's test is a test for the presence of reducing sugars, such as glucose or maltose, in a solution. The test involves adding Benedict's reagent, which is a blue solution of copper (II) sulfate, sodium carbonate, and sodium citrate, to the solution and heating it in a water bath. If reducing sugars are present, they reduce the copper (II) ions to copper (I) ions, which form a red, orange, or green precipitate of copper (I) oxide. ⁵
Explanation
Choice A rationale: Iodine is a chemical element that forms a brown solution of iodine and potassium iodide, known as iodine solution. When this solution is added to a sample that contains starch, it forms a complex with the starch molecules, which changes the color of the solution to blue-black. This is based on the fact that starch is a polysaccharide that has a helical structure, which can trap the iodine molecules inside. ²
Choice B rationale: Biuret is a chemical compound that forms a blue solution of copper (II) sulfate and sodium hydroxide, known as biuret reagent. When this reagent is added to a sample that contains proteins or peptides, it forms a complex with the copper (II) ions, which changes the color of the solution to violet or pink. This is based on the fact that proteins and peptides have peptide bonds, which have nitrogen atoms that can coordinate with the copper (II) ions. ³
Choice C rationale: Benedict's is a chemical compound that forms a blue solution of copper (II) sulfate, sodium carbonate, and sodium citrate, known as Benedict's reagent. When this reagent is heated with a sample that contains reducing sugars, such as glucose or maltose, it reduces the copper (II) ions to copper (I) ions, which form a red, orange, or green precipitate of copper (I) oxide. This is based on the fact that reducing sugars have free aldehyde or ketone groups that can donate electrons to the copper (II) ions. ⁴
Choice D rationale: Phenol red is a chemical compound that forms a red solution that is used as a pH indicator. When this solution is added to a sample that has an acidic or neutral pH, it remains red or turns yellow. When this solution is added to a sample that has an alkaline pH, it turns pink or fuchsia. This is based on the fact that phenol red has a sulfonated hydroxyquinone group that can lose or gain protons depending on the pH of the solution. ⁵
Choice E rationale: Sudan IV is a chemical compound that forms a red powder that is used as a stain for lipids. When this powder is dissolved in a solvent and added to a sample that contains lipids, such as fats or oils, it dissolves in the lipids and stains them red. When this solution is added to a sample that does not contain lipids, it remains in the solvent and does not stain the sample. This is based on the fact that Sudan IV is a nonpolar compound that can dissolve in nonpolar substances like lipids, but not in polar substances like water. ⁶.
Explanation
Choice A rationale: Sudan IV is a stain used to stain lipids. It is a red powder that dissolves in lipids and stains them red, but does not react with sugars. ¹
Choice B rationale: Benedict's is a reagent used to test for reducing sugars. It is a blue solution of copper (II) sulfate, sodium carbonate, and sodium citrate that reduces the copper (II) ions to copper (I) ions when heated with a reducing sugar, forming a red, orange, or green precipitate of copper (I) oxide. ⁴
Choice C rationale: Biuret is a reagent used to test for proteins and polypeptides. It is a blue solution of copper (II) sulfate and sodium hydroxide that forms a violet or pink complex with the peptide bonds in proteins or peptides. ⁸
Choice D rationale: Phenol red is a pH indicator. It is a red solution that changes color from yellow to red over the pH range 6.8 to 8.2, and from pink to fuchsia over 8.2 to 10.0. It does not react with sugars. ¹¹
Choice E rationale: Iodine is a reagent used to test for starch. It is a brown solution of iodine and potassium iodide that forms a blue-black complex with the starch molecules. It does not react with simple sugars. ¹⁴
If temperature is increased, what happens to the rate of diffusion?
Explanation
Choice A rationale: The rate of diffusion is the speed at which particles move from an area of high concentration to an area of low concentration. This depends on the temperature, the size of the particles, and the medium they are in. Temperature affects the kinetic energy and the speed of the particles, which in turn affects the frequency and intensity of their collisions. Higher temperatures mean higher kinetic energy and faster particles, which leads to faster diffusion. ³
Choice B rationale: The change in rate of diffusion is not unaffected by temperature. Temperature is one of the main factors that influences the rate of diffusion, as explained above. Therefore, this choice is incorrect.
Choice C rationale: Diffusion does not halt when temperature is increased. On the contrary, diffusion becomes faster when temperature is increased, as explained above. Therefore, this choice is incorrect.
Choice D rationale: The change in rate of diffusion is not unpredictable when temperature is increased. There is a clear relationship between temperature and diffusion, as explained above. Therefore, this choice is incorrect.
Choice E rationale: The rate of diffusion does not decrease when temperature is increased. On the contrary, diffusion becomes faster when temperature is increased, as explained above. Therefore, this choice is incorrect..
Explanation
Choice A rationale: The variant is a term used to describe a viral genome that may contain one or more mutations. It is not related to the difference in concentration between two areas. ³
Choice B rationale: The concentration gradient is the correct term for the difference in concentration between two areas. It is a measure of how steep the change in concentration is. ¹
Choice C rationale: Level gradient is not a term used in biology or chemistry. It may refer to the slope of a surface or a line, but not to the concentration of solutes in a solution.
Choice D rationale: The osmotic pressure is the minimum pressure required to prevent the flow of solvent molecules through a semipermeable membrane. It depends on the concentration of solute particles in the solution and is calculated with the formula π = iCRT. It is not the same as the concentration gradient, although it is related to it. ⁴
Choice E rationale: Turgid pressure is the force exerted by stored water against a cell wall. It is caused by the osmotic flow of water and occurs in plants, fungi, and bacteria. It is also called hydrostatic pressure and affects cell growth, movement, and dispersal. It is not the same as the concentration gradient, although it is influenced by it. ⁵
Explanation
Choice A rationale: Proteins contain nitrogen, but this is not the reason why they cannot pass through plasma membranes. Nitrogen is a common element in many organic molecules, including nucleic acids and amino acids, which can cross the membrane under certain conditions.
Choice B rationale: Proteins do not cause emulsification, which is the process of breaking down large fat droplets into smaller ones. Emulsification is facilitated by bile salts, which are amphipathic molecules that have both hydrophilic and hydrophobic regions. Proteins are not amphipathic, and they do not interact with fats in this way.
Choice C rationale: The membrane is made of protein, but this does not prevent proteins from passing through it. The membrane is composed of a phospholipid bilayer with embedded proteins, which can act as channels, carriers, receptors, or enzymes for various substances. Some proteins can cross the membrane by using these transport proteins, or by endocytosis or exocytosis.
Choice D rationale: Proteins are very large molecules, and this is the main reason why they cannot pass through plasma membranes. The size of a molecule determines its permeability across the membrane, and proteins are too big to diffuse through the small gaps between the phospholipids or the pores of the transport proteins. Proteins can only cross the membrane by vesicular transport, which requires energy and specific signals.
Choice E rationale: Proteins do not bind to the phospholipids, which are the main components of the membrane. Phospholipids are also amphipathic molecules, with a hydrophilic head and a hydrophobic tail. Proteins are generally hydrophilic, and they do not associate with the hydrophobic core of the membrane. Proteins can bind to other proteins or carbohydrates on the surface of the membrane, but this does not affect their ability to cross it.
Explanation
Choice A rationale: Density of media affects the rate of diffusion because it influences the frequency of collisions between the diffusing molecules and the molecules of the medium. The denser the medium, the slower the diffusion rate, and vice versa.
Choice B rationale: Size of molecules affects the rate of diffusion because it determines how easily the molecules can move through the spaces between the molecules of the medium. The smaller the molecules, the faster the diffusion rate, and vice versa.
Choice C rationale: Concentration gradient affects the rate of diffusion because it is the difference in concentration of the diffusing molecules between two regions. The higher the concentration gradient, the faster the diffusion rate, and vice versa.
Choice D rationale: Membrane permeability affects the rate of diffusion because it is the ability of the membrane to allow the diffusing molecules to pass through it. The more permeable the membrane, the faster the diffusion rate, and vice versa.
Choice E rationale: pH affects the rate of diffusion because it is the measure of acidity or alkalinity of the medium. pH can affect the charge and shape of the diffusing molecules, which can affect their ability to cross the membrane or interact with the molecules of the medium. pH can also affect the membrane permeability by altering the charge and shape of the membrane proteins.
Explanation
Choice A rationale: Low, high is incorrect because it is the opposite of the direction of simple diffusion. Simple diffusion is the passive movement of molecules along their concentration gradient, which means from high to low concentration.
Choice B rationale: Low, equal is incorrect because it is not the final state of simple diffusion. Simple diffusion will continue until the concentration of molecules is equal on both sides of the membrane.
Choice C rationale: Equal, low is incorrect because it is not possible for simple diffusion. Simple diffusion will stop when the concentration of molecules is equal on both sides of the membrane, and there will be no net movement of molecules.
Choice D rationale: Equal, high is incorrect because it is not possible for simple diffusion. Simple diffusion will stop when the concentration of molecules is equal on both sides of the membrane, and there will be no net movement of molecules.
Choice E rationale: High, low is correct because it is the definition of simple diffusion. Simple diffusion is the passive movement of molecules along their concentration gradient, which means from high to low concentration.
Small lipid soluble molecules would move through the plasma membrane by
Explanation
Choice A rationale: Diffusion is correct because it is the passive movement of molecules from an area of high concentration to an area of low concentration. Small lipid soluble molecules can easily cross the plasma membrane by diffusing through the hydrophobic core of the phospholipid bilayer.
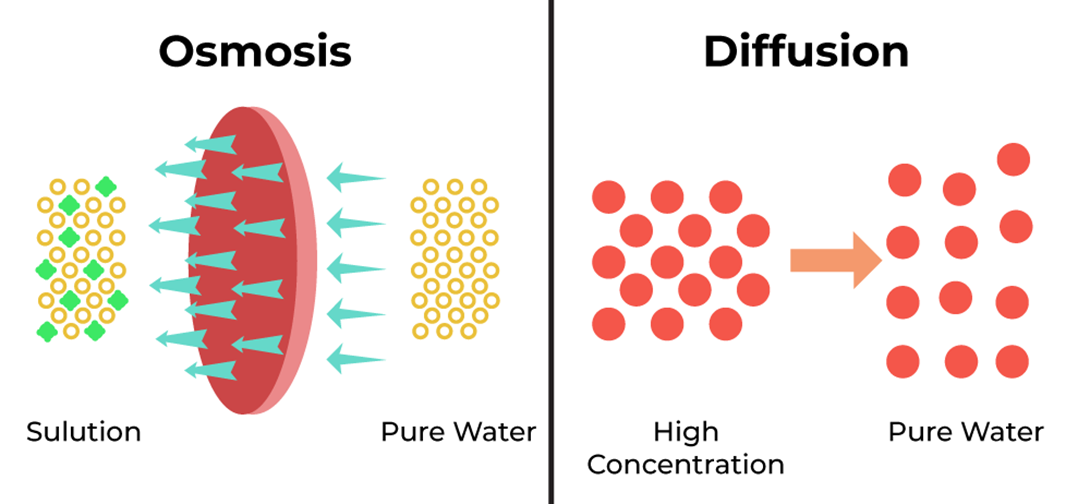
Choice B rationale: Filtration is incorrect because it is the process of separating solid particles from a fluid by passing it through a porous medium. Filtration does not involve the plasma membrane, and it does not depend on the solubility of the molecules.
Choice C rationale: Osmosis is incorrect because it is the diffusion of water across a selectively permeable membrane. Osmosis does not apply to lipid soluble molecules, which are not water molecules.
Choice D rationale: Active transport is incorrect because it is the movement of molecules across a membrane against their concentration gradient, which requires energy and transport proteins. Active transport does not depend on the solubility of the molecules, and it is not a passive process.
Choice E rationale: Pumping is incorrect because it is a type of active transport that involves the use of specific pumps to move ions or molecules across a membrane. Pumping does not apply to lipid soluble molecules, which are not ions or polar molecules.
Explanation
Choice A rationale: Movement of water into the blood from the dialysis solution is incorrect because it is not the main goal of dialysis. Dialysis aims to remove excess water and solutes from the blood, not to add more water to it. The dialysis solution is usually isotonic to the blood, which means it has the same osmotic pressure and does not cause water movement.
Choice B rationale: Simple diffusion across a semi-permeable membrane is correct because it is the process of dialysis. Dialysis is the separation of small molecules from large molecules by using a membrane that allows only the small molecules to pass through. The dialysis membrane is semi-permeable, which means it is selective in what it allows to cross. The dialysis solution contains a lower concentration of wastes than the blood, which creates a concentration gradient that drives the diffusion of wastes from the blood to the solution.
Choice C rationale: Active transport across a semi-permeable membrane is incorrect because it is not involved in dialysis. Active transport is the movement of molecules across a membrane against their concentration gradient, which requires energy and transport proteins. Active transport is not necessary for dialysis, since the concentration gradient is favorable for diffusion.
Choice D rationale: Active transport of glucose from the blood to the dialysis solution is incorrect because it is not beneficial for dialysis. Glucose is a vital nutrient for the body, and it should not be removed from the blood. The dialysis solution usually contains glucose to prevent its loss from the blood by diffusion.
Choice E rationale: Facilitated diffusion across a semi-permeable membrane is incorrect because it is not relevant for dialysis. Facilitated diffusion is the passive movement of molecules across a membrane with the help of transport proteins. Facilitated diffusion is not needed for dialysis, since the wastes are small enough to cross the membrane by simple diffusion.
After soaking for one hour in a solution of unknown concentration, a slice of potato appears to be very soft and limp. You can determine that the solution is
Explanation
Choice A rationale: A hypertonic solution has a higher solute concentration than the potato cell, which means it has a lower water potential. Water will move out of the potato cell by osmosis, causing it to shrink and become soft and limp.
Choice B rationale: A hypotonic solution has a lower solute concentration than the potato cell, which means it has a higher water potential. Water will move into the potato cell by osmosis, causing it to swell and become turgid and firm.
Choice C rationale: An isotonic solution has the same solute concentration as the potato cell, which means it has the same water potential. Water will move in and out of the potato cell at the same rate, causing it to remain unchanged in size and shape.
Choice D rationale: Tonic is not a valid term to describe the solute concentration of a solution. The correct terms are hypertonic, hypotonic, or isotonic.
Choice E rationale: I cannot determine anything without comparing multiple solutions is incorrect because the appearance of the potato slice after soaking in the solution provides enough information to determine the relative solute concentration of the solution.
Why do you think it is a good idea to soak wilted lettuce in cool water before serving it?
Explanation
Choice A rationale: An isotonic solution has the same solute concentration as the plant cells, which means it has the same water potential. Water will move in and out of the plant cells at the same rate, causing them to remain unchanged in size and shape. This will not help the lettuce to become less wilted.
Choice B rationale: A hypertonic solution has a higher solute concentration than the plant cells, which means it has a lower water potential. Water will move out of the plant cells by osmosis, causing them to shrink and become plasmolysed. This will make the lettuce more wilted and not crisper.
Choice C rationale: Soaking the lettuce in water would have an effect, depending on the relative solute concentration of the water and the plant cells. Water will move across the cell membrane by osmosis, either into or out of the plant cells, causing them to change in size and shape.
Choice D rationale: A hypotonic solution has a lower solute concentration than the plant cells, which means it has a higher water potential. Water will move into the plant cells by osmosis, causing them to swell and become turgid. This will make the lettuce crisper and more appealing.
Choice E rationale: A hypotonic solution will cause the plant cells to gain water and become turgid, not more wilted. Wilted lettuce is caused by the loss of water from the plant cells, which makes them flaccid and soft.
Explanation
Choice A rationale: The presence of chloroplasts is incorrect because chloroplasts are organelles that perform photosynthesis, not osmosis. Chloroplasts do not affect the water balance of the cell.
Choice B rationale: A plant cell will not burst in a hypotonic solution because water is moving out of the cell is incorrect because water moves into the cell in a hypotonic solution, not out of it. A hypotonic solution has a lower solute concentration than the cell, so water flows from the solution to the cell by osmosis.
Choice C rationale: Chloroplasts that help pump the excess water out of the cell is incorrect because chloroplasts do not have any role in pumping water out of the cell. The cell uses active transport to pump out excess water, which requires energy from ATP, not chloroplasts.
Choice D rationale: The plasma membrane is incorrect because the plasma membrane is permeable to water, so it cannot prevent water from entering the cell. The plasma membrane only regulates the passage of solutes, not water.
Choice E rationale: The cell wall is correct because the cell wall is a rigid structure that surrounds the plasma membrane and provides mechanical support to the cell. The cell wall can withstand the pressure of water entering the cell and prevent the cell from bursting. The cell wall is made of cellulose, a polysaccharide that is resistant to water.
A(n) ____ solution has a lower concentration of water than the cell placed in the solution.
Explanation
Choice A rationale: Osmotic is incorrect because osmotic is an adjective that describes the movement of water across a semipermeable membrane, not a type of solution. Osmosis is the process by which water moves from a region of high water concentration to a region of low water concentration.
Choice B rationale: Isotonic is incorrect because isotonic is a type of solution that has the same concentration of water as the cell placed in the solution. In an isotonic solution, there is no net movement of water across the cell membrane.
Choice C rationale: Hypertonic is correct because hypertonic is a type of solution that has a lower concentration of water than the cell placed in the solution. In a hypertonic solution, water moves out of the cell and into the solution by osmosis, causing the cell to shrink.
Choice D rationale: Diffusive is incorrect because diffusive is an adjective that describes the movement of molecules from a region of high concentration to a region of low concentration, not a type of solution. Diffusion is the process by which molecules move across a membrane or a space due to their random motion.
Choice E rationale: Hypotonic is incorrect because hypotonic is a type of solution that has a higher concentration of water than the cell placed in the solution. In a hypotonic solution, water moves into the cell and out of the solution by osmosis, causing the cell to swell.
Sign Up or Login to view all the 46 Questions on this Exam
Join over 100,000+ nursing students using Nursingprepexams’s science-backend flashcards, practice tests and expert solutions to improve their grades and reach their goals.
Sign Up Now

